???????????, ??? ????????? ???????????.
?????????? ??????
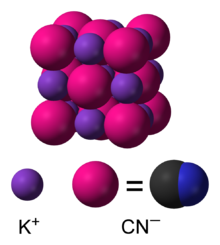
|
| Names
|
| IUPAC name
Potassium cyanide
|
| Identifiers
|
|
|
|
|
|
|
| ChEBI
|
|
| ChemSpider
|
|
| ECHA InfoCard
|
100.005.267

|
| EC Number
|
|
|
|
|
| RTECS number
|
|
| UNII
|
|
| UN number
|
1680
|
|
|
|
| InChI
|
|
| SMILES
|
|
| Properties
|
| ????????? ??????
|
|
| Molar mass
|
0 g mol
?1
|
| Appearance
|
White crystalline solid
deliquescent
|
| Odor
|
faint,
almond
-like
|
| ????????
|
1.52 g/cm
3
|
| ??????????
|
|
| ??????????
|
|
|
|
71.6 g/100 ml (25 °C)
100 g/100 mL (100 °C)
|
| Solubility
in
methanol
|
4.91 g/100 mL (20 °C)
|
| Solubility
in
glycerol
|
soluble
|
| Solubility
in
formamide
|
14.6 g/100 mL
|
| Solubility
in
ethanol
|
0.57 g/100mL
|
| Solubility
in
hydroxylamine
|
41 g/100 mL
|
| ????????
(p
K
a
)
|
11.0
|
|
|
−37.0·10
?6
cm
3
/mol
|
| Refractive index
(
n
D
)
|
1.410
|
| Thermochemistry
|
Std enthalpy of
formation
Δ
f
H
o
298
|
−131.5 kJ/mol
|
Standard molar
entropy
S
o
298
|
127.8 J K
−1
mol
−1
|
| Hazards
|
| Safety data sheet
|
ICSC 0671
|
| GHS pictograms
|
   
|
| GHS Signal word
|
Danger
|
|
|
H290
,
H300
,
H310
,
H330
,
H370
,
H372
,
H410
|
|
|
P260
,
P264
,
P273
,
P280
,
P284
,
P301+310
|
| Flash point
|
{{{value}}}
|
| Lethal dose or concentration (LD, LC):
|
|
|
5 mg/kg (oral, rabbit)
10 mg/kg (oral, rat)
5 mg/kg (oral, rat)
8.5 mg/kg (oral, mouse)
[1]
|
| NIOSH
(US health exposure limits):
|
|
|
TWA 5 mg/m
3
|
|
|
C 5 mg/m
3
(4.7 ppm) [10-minute]
|
|
|
25 mg/m
3
|
| Related compounds
|
| Other
anions
|
Potassium cyanate
Potassium thiocyanate
|
| Other
cations
|
Sodium cyanide
|
Except where otherwise noted, data are given for materials in their
standard state
(at 25 °C [77 °F], 100 kPa).
|
KCN ????? ????????????? ??? ???????????
?????????? ?????? (Potassium cyanide).
??????????? ??????????, ??????? ???? ??????? ??????????, ????????????,
??????????????????????
??? ??????? ???. ?????????????????, ?????????????
??????????????????????
?????????????????.
[3]
?????????? ?????? ??? ???????????. ?????????
???????????????
??? ??????????
??????? ??????
????????????. ?????????? ????????? ?????? ?????.
[4]
???????? ? ??? ????????????? ??????, ??? ?????????? ??? ???????????.
[5]
?????????????? ???????? ????????? ?????????? ??????????? ?????????? ????????.
[6]
??????? ??????
?????????? ????????????????
??????????? ???????????????????? ????????? ? ????? ??????????????? ?????????? ?????? ??????????????:
[7]
- HCN + KOH → KCN + H
2
O
?????????? ????????? ?????????? ???????????????? ??????????????????
- HCONH
2
+ KOH → KCN + 2H
2
O
????????? ??????? 50,000 ?? ?????????? ?????? ???????????????.
??????? ?????????
[
?????????
]
1900 ?? ????? ?????????? ???? ?????????????????????
??????? ??????????
???????? ???????????? ??????? ?????? ????????? ?????????? ?????? ?????????.
[3]
? ??????? ?????????? ??????????? ???????????????? ?????????? ?????? ???????????????????????:
[8]
K
4
[Fe(CN)
6
] → 4 KCN + FeC
2
+ N
2
?????????? ??????? ??????
[
?????????
]
?????????? ??????
????????????
???????? ????????
???????????????
??????????? ?? ????????????? ?????????????????, ????????????? ????????????? ???????? ?????????? ??????????. ??? ?????????? ????????? ?????? ???????????? ?????? ?????????????????????? ?????? ???????? ????????.
???????? ?????????
????????
????????????????
????????????. ???????????, ??????? ?????? ??????, ???????????????????????? ???? ???????????????? ????????????. ??????????
???????
?????????? ??????????? ?????????????????? ?????? ??????????????. ??????????, ?????? ?????? ?????????? ??? ????????. ??????????????? ????????? ????????? ???? ?????????????????????? ????? ?????? ??????????????????? ???????????????. ????????????? ???? ?????????????? ??????? ?????????? ???? ????????????? ??????????. ? ???????? ??????????????????????? ?????? ?????????????????? ??????????????????.
???????? ?????????
???? ???? ?????????????. ?????????? ?????????? ???????????????? ????????????? ?????? ???? 200?300 ??.????????.
?????????????? ???????
[
?????????
]
??????
????????? ????????? ????????????????
|
| HCN
|
|
|
|
He
|
| LiCN
|
Be(CN)
2
|
B
|
C
|
NH
4
CN
|
OCN
?
,
-NCO
|
FCN
|
Ne
|
| NaCN
|
Mg(CN)
2
|
Al(CN)
3
|
Si(CN)4
,
Me
3
SiCN
|
P(CN)
3
|
SCN
?
,
-NCS
,
(SCN)
2
,
S(CN)
2
|
ClCN
|
Ar
|
| KCN
|
Ca(CN)
2
|
Sc(CN)
3
|
Ti(CN)
4
|
Cr(CN)
6
4?
|
Cr(CN)
6
3?
|
Mn(CN)
2
|
Fe(CN)
3
,
Fe(CN)
6
4?
,
Fe(CN)
6
3?
|
Co(CN)
2
,
Co(CN)
3
|
Ni(CN)
2
Ni(CN)
4
2?
|
CuCN
|
Zn(CN)
2
|
Ga(CN)
3
|
Ge
|
As(CN)
3
|
SeCN
?
(SeCN)
2
Se(CN)
2
|
BrCN
|
Kr
|
| RbCN
|
Sr(CN)
2
|
Y(CN)
3
|
Zr(CN)
4
|
Nb
|
Mo(CN)
8
4?
|
Tc
|
Ru(CN)
6
3?
|
Rh(CN)
6
3?
|
Pd(CN)
2
|
AgCN
|
Cd(CN)
2
|
In(CN)
3
|
Sn
|
Sb(CN)
3
|
Te
|
ICN
|
Xe
|
| CsCN
|
Ba(CN)
2
|
|
Hf
|
Ta
|
W(CN)
8
4?
|
Re
|
Os(CN)
6
3?
|
Ir(CN)
6
3?
|
Pt(CN)
4
2-
,
Pt(CN)
6
4-
|
AuCN
,
Au(CN)
2
?
|
Hg
2
(CN)
2
,
Hg(CN)
2
|
TlCN
|
Pb(CN)
2
|
Bi(CN)
3
|
Po
|
At
|
Rn
|
| Fr
|
Ra
|
|
Rf
|
Db
|
Sg
|
Bh
|
Hs
|
Mt
|
Ds
|
Rg
|
Cn
|
Nh
|
Fl
|
Mc
|
Lv
|
Ts
|
Og
|
|
|
↓
|
|
|
| La
|
Ce(CN)
3
,
Ce(CN)
4
|
Pr
|
Nd
|
Pm
|
Sm
|
Eu
|
Gd(CN)
3
|
Tb
|
Dy
|
Ho
|
Er
|
Tm
|
Yb
|
Lu
|
| Ac
|
Th
|
Pa
|
UO
2
(CN)
2
|
Np
|
Pu
|
Am
|
Cm
|
Bk
|
Cf
|
Es
|
Fm
|
Md
|
No
|
Lr
|
|
|---|
???????? ???????????
·
???????? ??????????????????
·
????????
·
???????????
·
?????????????? (??????????????)
·
?????????? ????????
·
????????????
·
?????????
·
???????
·
???? ????????
·
??????????
·
????????? ????????? (????????? ????????)
·
??????????????
·
???????
·
??????? ??????????
·
??????
·
???????????
·
??????
·
??????, 2,4,6-?????????-
·
???????? ???????????????
·
?????????? ?
·
ANTU (
ANTU (????-???????????????)
·
???????? ????????????
·
???????? ??????????? (??????? ???????)
·
??????? ????????????
·
?????
·
?????????-????
·
?????????-?????
·
????? ????????
·
??????????, 3- (???????????????) -
·
?????????????? ?????
·
????????????, 4,5-???????? -2- (???????????????) -
·
?????????????????
·
?????? ????????
·
?????? ??????
·
???????? (2.2.1) ?????????-2-????????????
·
????(???????????) ???????
·
?????????????????
·
????? ????????????
·
????? ????????????
·
????? ???????????? ??????? ??????????? ????????
·
?????????????
·
???????
·
???????? ???????
·
???????? ?????????????
·
??????? ??????????
·
??????????
·
???????????
·
????????? ????????
·
????????|??????????
·
????? ????????
·
????????????????
·
??????????
·
???????????????
·
???????
·
???????????
·
?????????????? ????????
·
??????????????? ?????
·
2-?????????????
·
?????????? ???????????????
·
?????????
·
??????????? ???
·
??????????? ????? ???
·
?????????????
·
?????????????
·
???????????????
·
???????? ????????
·
????????? ???????
·
?????????
·
????????
·
Cresol, -o
·
?????????
·
???????????????
·
??????? ????????
·
??????? ???????
·
????????
·
???????? ????????
·
????????????????
·
?????????????????
·
???????????|?????????? (14)
·
????????
·
????????-???-?????
·
????????
·
??????? (??????)
·
???????????? ???
·
?????????????????????????
·
???????????
·
????????????
·
?????????????????
·
?????? ????????????????
·
???????????
·
??????????? ???
·
?????????
·
??????????
·
????????????
·
??????????????????????
·
????????????????
·
???????????
·
???????????????
·
????????
·
???????????
·
??????????????
·
?????????
·
???????????
·
???????????? ???????
·
??????????????????
·
?????????
·
????????????
·
????????
·
????????????????
·
??????????????
·
??????????? ???????????
·
????????
·
???????????
·
?????? ?????????????
·
?????? ???????
·
??????????????
·
??????????
·
???????????????
·
??????????
·
???????????????
·
??????????????
·
???????????
·
???????
·
???????
·
?????????????????
·
??????????????? ?????
·
?????????????? ????????
·
???????????
·
????????
·
????????????|????????????
·
???????????? ???????????
·
??????????????? ??????????????
·
????????????
·
????????????
·
?????????????
·
?????????????
·
???????
·
?????? ????????????
·
??????????????????????????
·
?????????
·
??????? ??????|????????????? ?????
·
??????? ???????? (????? ??????)
·
??????? ????????
·
??????? ??????????
·
??????? ????????
·
??????? ??????
·
?????????????
·
??? ??????????????
·
?????????
·
????????
·
???????? ????????????
·
?????????????
·
????????????
·
??????????
·
????????
·
?????? ????????
·
????????????
·
???????????????
·
??????????? ??????????
·
??????????? ????????
·
??????????? ???????
·
???????????? ?????????????
·
????????????? ???????????
·
??????????????????
·
????????????? ????????
·
??????????????????????? ???????????
·
?????????????
·
???????????? ????????
·
???????????????
·
?????????????
·
?????????
·
?????????????? ??????????? ??????????
·
????? 2-?????????????????
·
????? ????????
·
????? ???????????????
·
????? ?????????
·
????? ???????????
·
????? ?????????????????
·
????? ????????????
·
????? ?????????? ??????????
·
????? ???????????
·
????? ????? ???????
·
????????????? ??????????
·
????????????????????
·
????????????
·
?????????
·
???????????????
·
??????????? ??
·
??????????????
·
????????
·
?????????? ??????
·
?????? ????????
·
???????????
·
???????????|??????????? ????????
·
???????? ???????
·
????????????
·
?????????????????????
·
??????? ?????????|??????? ?? ???????
·
??????????
·
??????????? ??????????
·
??????
·
????????
·
???????????????
·
???????????
·
??????????? ??????????????
·
???????????
·
???????????-?????
·
?????? ?????
·
??????????
·
??????????????
·
???????????? ?????
·
?????????????????????????
·
?????|?????
·
????? ?????????????
·
?????????????? ??????????????
·
?????????????? ??????????
·
?????????????
·
??????????????
·
????????
·
???????|???????
·
??????????
·
??????????
·
???????
·
????????
·
???????? ?????????????
·
???????? ?????????????
·
???????? ????????????
·
???????????????
·
???????????????, ???????????? (1:1)
·
?????????????
·
???????????
·
????????????
·
???????? -210
·
?????????? ??????????
·
?????????? ??????
·
?????????? ????? ??????
·
??????????
·
??????????? ????????
·
?????????????????
·
?????????????
·
???????????????, 3-??????
·
?????????????, 4 ?????
·
?????????????
·
????????????
·
?????
·
???????, 4 ?????
·
???????, 4 ??????-, 1 ???????
·
?????????
·
???????
·
????????
·
????
·
????????? ?????
·
????????????? ??????????????
·
Silane, (4-aminobutyl)diethoxymethyl-
·
?????? ??????????
·
?????? ?????
·
?????????? ?????|?????? ?????????????
·
?????? ??????
·
?????? ?????????????????
·
?????? ???????????????????
·
?????? ?????????
·
?????? ?????????
·
???????????, ????????????????????-
·
???????????
·
??????????? ????????
·
??????????
·
??????????, 3-????????????? ????????
·
???? ??????????|???? ?? ???????
·
???? ???????????????
·
???? ???????????
·
?????????? ?????
·
????
·
??????????
·
?????????? ???????????????
·
TEPP
·
?????????
·
?????? ???? ????
·
??????????????
·
???????????????????
·
?????? ????????
·
????? ??????????
·
????? ????????
·
????? ?????????
·
????? ????????
·
????????????
·
???????????
·
????????
·
????????
·
??????????????????
·
?????????? ??????????????
·
???????????
·
???????????
·
Trichloro(chloromethyl)silane
·
Trichloro(dichlorophenyl)silane
·
?????????????????? ????????
·
?????????????????????
·
????????????????
·
??????????????????????
·
?????????????????
·
??????????????????????
·
??????????????????????? ??????????
·
?????????????????? ????????
·
?????????????? ????????
·
Tris(2-chloroethyl)amine
·
???????????
·
????? ?????????? ??????
·
???????
·
??????? ??????
·
????? ??????????
·
?????? ????????
·
|