From Wikipedia, the free encyclopedia
Chemical compound
Trirhenium nonachloride
is a
compound
with the formula ReCl
3
, sometimes also written Re
3
Cl
9
. It is a dark red hygroscopic solid that is insoluble in ordinary solvents. The compound is important in the history of
inorganic chemistry
as an early example of a cluster compound with metal-metal bonds.
[1]
It is used as a starting material for synthesis of other rhenium complexes.
Structure and physical properties
[
edit
]
As shown by
X-ray crystallography
trirhenium nonachloride consists of Re
3
Cl
12
subunits that share three chloride
bridges
with adjacent clusters. The interconnected network of clusters forms sheets. Around each Re center are seven ligands, four bridging chlorides, one terminal chloride, and two Re-Re bonds.
[2]
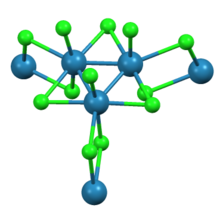 Re
3
Cl
12
cluster within ReCl
3
, shown with full coordination sphere around each chloride.
Re
3
Cl
12
cluster within ReCl
3
, shown with full coordination sphere around each chloride.
The
heat of oxidation
is evaluated according to the equation:
- 1/3 Re
3
Cl
9
+ 4 OH
?
+ 2 OCl
?
→ ReO
4
?
+ 2 H
2
O + 5Cl
?
The enthalpy for this process is 190.7 ± 0.2 kcal/mol.
[2]
Preparation and reactions
[
edit
]
The compound was discovered in 1932, although these workers did not determine its structure, which is unusual for metal chlorides.
[3]
Trirhenium nonachloride is efficiently prepared by thermal decomposition of
rhenium pentachloride
or hexachlororhenic(IV) acid:
[4]
- 3 ReCl
5
→ Re
3
Cl
9
+ 3 Cl
2
If the sample is vacuum
sublimed
at 500 °C, the resulting material is comparatively unreactive, but the partially hydrated material can be more useful synthetically. Other synthetic methods include treating
rhenium
with
sulfuryl chloride
. This process is sometimes conducted with the addition of
aluminium chloride
.
[2]
It is also obtained by heating Re
2
(O
2
CCH
3
)
4
Cl
2
under HCl:
- 3/2 Re
2
(O
2
CCH
3
)
4
Cl
2
+ 6 HCl → Re
3
Cl
9
+ 6 HO
2
CCH
3
Reaction of the tri- and pentachlorides gives
rhenium tetrachloride
:
- 3 ReCl
5
+ Re
3
Cl
9
→ 6 ReCl
4
References
[
edit
]
|
|---|
| Rhenium(0)
| |
|---|
| Rhenium(I)
| |
|---|
| Rhenium(II)
| |
|---|
| Rhenium(III)
| |
|---|
| Rhenium(IV)
| |
|---|
| Rhenium(V)
| |
|---|
| Rhenium(VI)
| |
|---|
| Rhenium(VII)
|
|
|---|
Salts and covalent derivatives of the
chloride
ion
|
|---|
|