From Wikipedia, the free encyclopedia
Curium(III) chloride

|
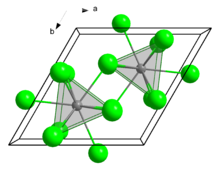
Crystal structure
|
| Identifiers
|
|
|
|
|
|
|
|
|
|
InChI=1S/3ClH.Cm/h3*1H;/q;;;+3/p-3
Key: PTLGMSBPLOHNBD-UHFFFAOYSA-K
|
|
|
| Properties
|
|
|
Cl
3
Cm
|
| Molar mass
|
353
g·mol
?1
|
| Appearance
|
White solid (anhydrous)
Light green solid (hydrate)
|
| Melting point
|
695 °C (1,283 °F; 968 K)
[
citation needed
]
|
Except where otherwise noted, data are given for materials in their
standard state
(at 25 °C [77 °F], 100 kPa).
|
Chemical compound
Curium(III) chloride
is the chemical compound with the formula CmCl
3
.
Structure
[
edit
]
Curium(III) chloride has a 9 coordinate
tricapped trigonal prismatic geometry
.
[1]
Synthesis
[
edit
]
Curium(III) chloride can be obtained from the reaction of
hydrogen chloride
gas with
curium dioxide
,
curium(III) oxide
, or
curium(III) oxychloride
at a temperature of 400-600 °C:
- CmOCl + 2HCl → CmCl
3
+ H
2
O
It can also be obtained from the dissolution of metallic curium in dilute
hydrochloric acid
:
[2]
- 2Cm + 6HCl → 2CmCl
3
+ 3H
2
This method has a number of disadvantages associated with the ongoing processes of hydrolysis and hydration of the resulting compound in an aqueous solution, making it problematic to obtain a pure product using this reaction.
It can be obtained from the reaction of
curium nitride
with
cadmium chloride
:
[3]
- 2 CmN + 3 CdCl
2
→ 2 CmCl
3
+ Cd
3
N
2
References
[
edit
]
- ^
Greenwood, N. N.; Earnshaw, A. (1997).
Chemistry of the Elements
(2nd ed.). Butterworth, UK. p. 1270.
{{
cite book
}}
: CS1 maint: location missing publisher (
link
)
- ^
Wallmann, J. C.; Fuger, J.; Peterson, J. R.; Green, J. L. (1 November 1967).
"Crystal structure and lattice parameters of curium trichloride"
.
Journal of Inorganic and Nuclear Chemistry
.
29
(11): 2745?2751.
doi
:
10.1016/0022-1902(67)80013-7
.
ISSN
0022-1902
.
S2CID
97334114
. Retrieved
3 July
2023
.
- ^
Hayashi, Hirokazu; Takano, Masahide; Otobe, Haruyoshi; Koyama, Tadafumi (July 2013). "Syntheses and thermal analyses of curium trichloride".
Journal of Radioanalytical and Nuclear Chemistry
.
297
(1): 139?144.
doi
:
10.1007/s10967-012-2413-7
.
S2CID
95792512
.
|
|---|
| Curium(III)
| |
|---|
| Curium(IV)
| |
|---|
| Curium(VI)
| |
|---|
Salts and covalent derivatives of the
chloride
ion
|
|---|
|